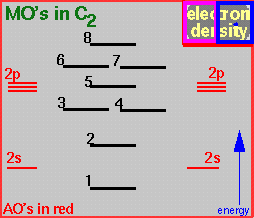
The electron density is available with two different contour values, by clicking in the left (pink) or right (blue) half of the 'electron density area' (upper right corner) in the MO energy plot.
What is the number of the highest occupied orbital (HOMO)?
What is the bond order in the molecule?
Which bonding MO's have contributions from the 2p AO's perpendicular to the C-C axis?
Why is the blue bonding part of MO 5 smaller than expected for 2p-2p overlap?
Explain the hollow shape of the electron density surface. (Look at the high density contour.)