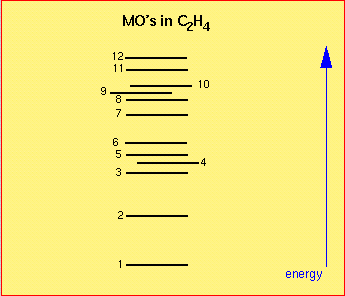
Questions on C2H4, ethene, ethylene:
Which occupied MO's are bonding w.r.t. the C-C bond?
Which ones are anti-bonding?
Which carbon 2p orbitals contribute to MO 3?
Which MO's only have contributions from 2px orbitals?
| Select an orbital for viewing. Click on a line in the scheme. | ||

|
||
| Orbital solid , translucent , off | ||
|
Definition of axes: x is perpendicular to (Ned.: loodrecht op) the plane of molecule, y is in the direction of the C-C bond. (show/hide)
Questions on C2H4, ethene, ethylene:
Which occupied MO's are bonding w.r.t. the C-C bond? | ||